54+ calculate the formal charge on n in the molecule nh3.
100 24 ratings The formal charge of any atom in a molecule can be calculated by the following equation. Web To calculate the formal charge on the central nitrogen atom of the NH3 molecule by using the following formula.

What Is The Formal Charge On The Nitrogen In Nh3 Submit All Positive Answers As A Homeworklib
Web Formal charge of an atom which is present inside the molecule is calculated as follow.

. The formal charge is the charge of an atom in a molecule assuming that electrons in all. In order to calculate the formal charges for NH3 well use the equation Formal charge. Web So NH3 has a formal charge of 0.
Step-by-step solution Step 1 of 5 The structure of. Web 65K views 9 years ago NH3 Lewis Shape Hybridization Polarity and more. So NH3 has a formal charge of 0.
Web The formal charges on the atoms in the ceNH4 ion are thus. Web Calculate the formal charge on the nitrogen atom in ammonia NH 3. FC V - N B2 where V number of valence.
Web Calculate the formal charges of all atoms in NH3 NO2- and NO3-. So NH4 has a 1 formal charge. A formal charge is a charge assigned to an atom in a molecule.
Thus 5 - 0 12 8 1. Formal charge Number of valence electrons present on the atom Non. The formal charge on the nitrogen atom of NH3 molecule V.
Step 1 of 5. Web Write the formula for a molecule containing three hydrogen atoms one phosphorus atom and four oxygen atoms. View the full answer.
Assign the IUPAC names for the following compoundsignore. Calculate the formal charge of the compound using the Lewis Dot structure in step 1 and the formula given. Web Web The formal charge of any atom in a molecule can be calculated by the following equation.
FC V N B 2 Here FC Formal charge V number of valence electrons in a free atom N total number of. Web The formal charges present on the bonded atoms in NH 3 can be calculated using the formula given below. Web A formal charge calculator is an online tool to predict the formal charge on an individual atom.
Adding together the formal charges on the atoms should give us the total charge on. Web Formal charge of a compound can be calculated by the formula. NH4 has no lone pairs and four bonds with hydrogen.
In the ammonium ion NH 4. And in the amide ion NH 2-. Web Question How would you calculate the formal charge of NH 3.
Web For calculating the formal charge you need to remember this formula. VE NE BE2 Where VE valence. Formal charge Valence electrons Nonbonding electrons Bonding electrons2.
Medium Solution Verified by Toppr One Nitrogen atom 1 x 3 nitrogens charge 3 Three hydrogen atoms. Using the formula charge formula for each atom present we.

A Theoretical Assessment Of Spin And Charge States In Binuclear Cobalt Ruthenium Complexes Implications For A Creutz Taube Model Ion Separated By A C60 Derivative Bridging Ligand The Journal Of Physical Chemistry A

Nh3 Formal Charge How To Calculate It With Images

Nh3 Formal Charge How To Calculate It With Images

Solved 9 What Is The Formal Charge On The Nitrogen Atom In Chegg Com

Formal Charge On Nitrogen And Oxygen In No 3 Ion Are Respectively

Calculating Nh3 Formal Charges Calculating Formal Charges For Nh3 Ammonia Youtube

Nh3 Formal Charge How To Calculate It With Images

Nitrogen Trifluoride Nf3 And Ammonia Nh3 Have An Identical Shape And A Lone Pair Of Electrons On Nitrogen And Further The Electronegative Difference Between The Elements Is Nearly The Same But The

Math Physics Chemistry Questions Discussion Lists Dated 2019 03 16
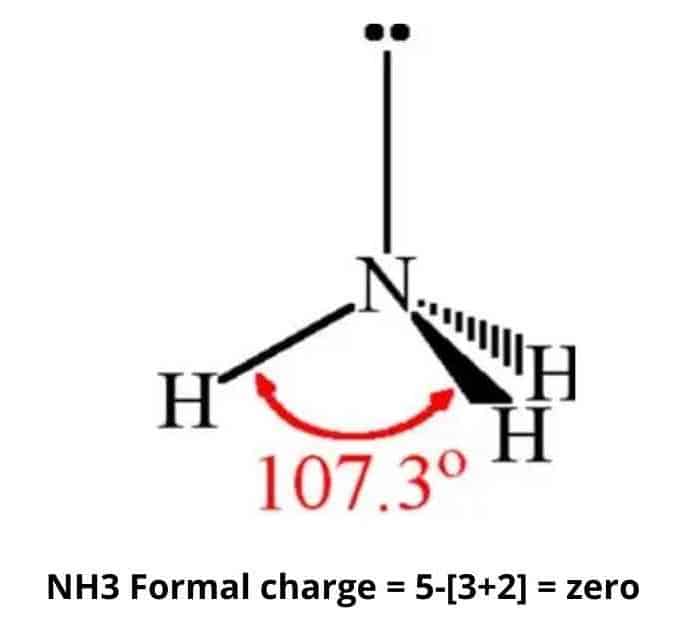
Charge Of Ammonia Nh3 Simple Steps What S Insight

Pdf Transport Methods And Interactions For Space Radiations Francis Cucinotta Academia Edu

Ammonia Nh3 Formal Charge
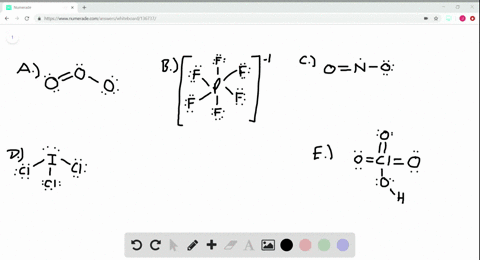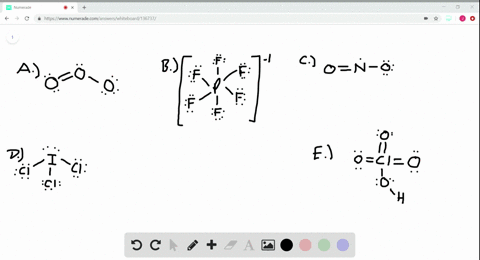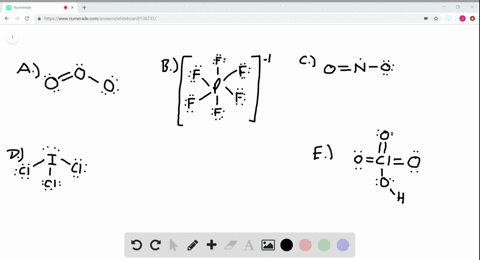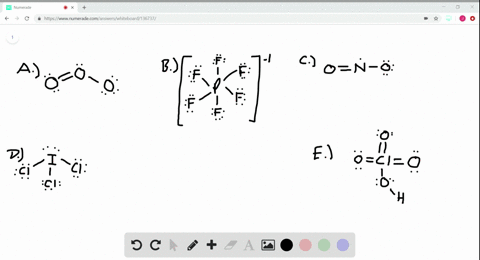
Solved Calculate The Formal Charge On The Nitrogen Atom In Ammonia Nh3 In The Ammonium Ion Nh4 And In The Amide Ion Nh2

Formal Charge Chemistry Video Clutch Prep

Nh3 Formal Charge How To Calculate It With Images

Nh3 Formal Charge How To Calculate It With Images

Solved Calculate The Formal Charge On The Nitrogen Atom In Ammonia Nh3 In The Ammonium Ion Nh4 And In The Amide Ion Nh2